Haddy Research Group

Research in the Haddy lab is on the catalytic production of molecular oxygen by photosystem II (PSII) during photosynthesis by plants and cyanobacteria. The evolution of O2 occurs as a result of the biological conversion of solar energy into chemical energy. Because of its key role in the natural energy cycle of the earth’s creatures, catalytic O2 production in plants is an important model for artificial solar energy systems. Oxygen is produced at a catalytic Mn4CaO5 cluster that is served by key amino acid residues, inorganic ion cofactors, and channels for the movement of reactant H2O molecules and product H+ and O2 molecules. In our research, we are interested in how this complex catalytic reaction is promoted by two nearby Cl– ions cofactors and the Ca2+ ion of the Mn4CaO5 cluster. We combine biochemical techniques such as protein purification and enzyme kinetics assays with physical techniques, particularly electron paramagnetic resonance (EPR) spectroscopic.
Research

Research on PSII in the Haddy lab often makes use of electron paramagnetic resonance (EPR) spectroscopy to characterize the sites that participate in electron transfer. EPR spectroscopy requires the presence of unpaired electrons and detects transitions between spin states imposed by a magnetic field. Two major types of biological centers can be studied with this technique: organic radicals, which often involve aromatic ring systems as in tyrosine, tryptophan, or quinones; and paramagnetic transition metal complexes, which commonly involve iron, copper, or manganese ions. EPR spectroscopy is ideal for studying biological electron transfer processes, such as those that take place in photosystem II, because the sites of electron transfer often involve the formation of organic radicals or changes in metal oxidation states.
In photosystem II, EPR spectroscopy can be used to observe the Mn4CaO5 cluster and the nearby tyrosine radical, Tyr Z, which accepts electron from the cluster. The signals can be used to assess how activators and inhibitors affect the catalytic cycle during oxygen production and the electron transfer steps related to it. By combining enzyme kinetics analyses with EPR spectroscopy, the effects of Cl– and Ca2+ may be understood at a molecular level.
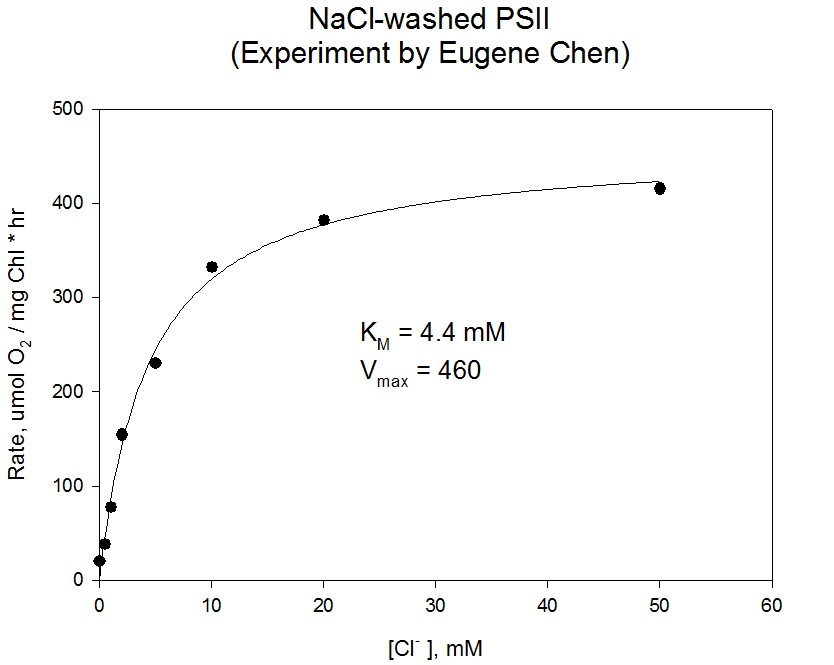
Chloride and calcium are important inorganic cofactors in the photosynthetic production of molecular oxygen by photosystem II. The Ca2+ ion, which is directly bound to the Mn4CaO5 cluster, has a critical role in directing electrons into the electron transfer chain via the Tyr Z residue. The Cl– ion, which is more distantly bound, is required for the Mn4CaO5 cluster to reach the higher oxidation states. Each ion influences the coupling of the Mn in the cluster, as observed using electron paramagnetic resonance (EPR) methods. In addition, each ion participates in hydrogen bond networks involved in the movement of protons or substrate water molecules.
Our studies combine enzyme kinetics methods and EPR spectroscopy to better understand the roles of Cl– and Ca2+ in the production of oxygen by PSII. Using enzyme kinetics studies, we have characterized the function of Cl– by replacing it with anions that can activate or inhibit O2 evolution. For example, studies of inhibition kinetics have characterized fluoride and azide anions as competitive inhibitors of chloride activation. Iodide and nitrite have been found to be activators at low concentrations but inhibitors at higher concentrations. Other enzyme kinetics studies have examined the Ca2+ requirement of PSII, its dependence on Cl– as a co-activator, and the effects of decreased pH.
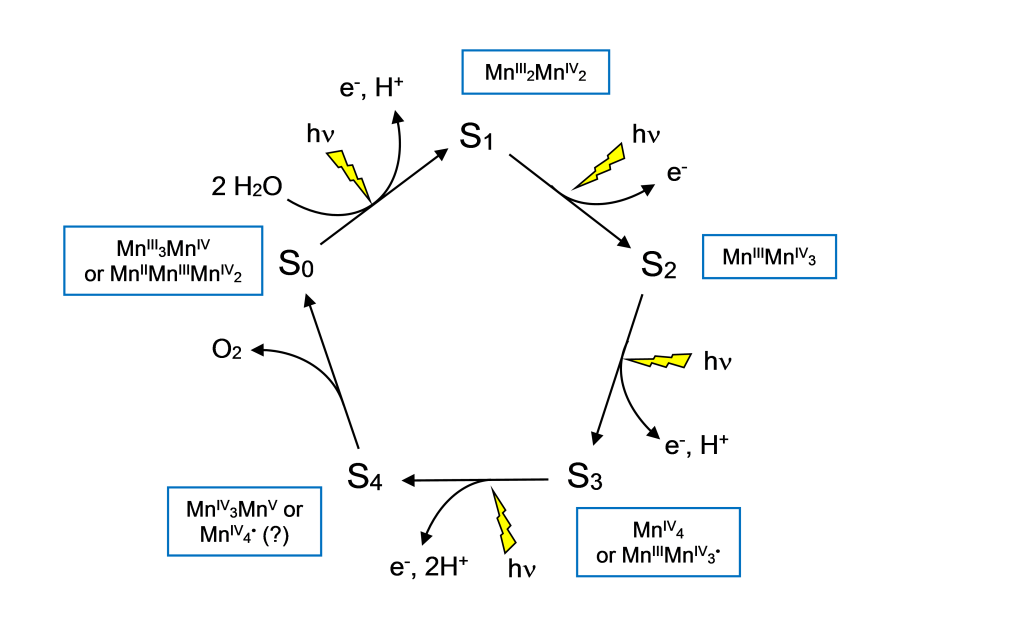
Photosystem II is one of the two major light absorbing protein complexes that absorb light energy to drive electron transfer processes required for the formation of high energy, biochemically useful molecules such as ATP. In photosystem II, H2O provides electrons to the electron transfer chain through a catalytic Mn4CaO5 cluster, with O2 released as a byproduct. The Mn4CaO5 cluster passes through five oxidation states during the catalytic cycle that produces O2. Each advancement in oxidation state is driven by the absorption of a photon at the PSII reaction center, a special cluster of chlorophyll molecules. This process enables the four-electron conversion of H2O to O2, which is unique among biochemical reactions and has been highly conserved across all species that carry out oxygenic photosynthesis.
In addition to the Mn4CaO5 cluster, the evolution of O2 is facilitated by two nearby Cl– ions, numerous coordinating amino acid residues, and a redox active tyrosine residue (Tyr Z) that passes electron from the Mn4CaO5 cluster to the reaction center. Much is known about the structure of PSII and the oxidation states of Mn during the catalytic cycle, based on a wide body of research using electron paramagnetic resonance (EPR), X-ray absorption, and high-resolution X-ray crystallography studies. However much remains to be understood about the mechanism by which H2O is converted into O2 and how O2 and protons are released from the complex. Our studies have the eventual goal of leading to a better understanding of how the Ca2+ and Cl– ions affect the function of the Mn4CaO5 cluster in the catalytic cycle that produces O2.