People
Kimberly Petersen

Field: Organic Chemistry, Asymmetric Methodology Development
Room: 411 Sullivan Science Building
Phone: 336.334.4271
Email: kspeters@uncg.edu
Research Website: https://chem.uncg.edu/petersen/
Education
B.S. Chemistry, University of Wisconsin-Madison, 2000
M.S., Chemistry, American University, 2004
Ph.D., Organic Chemistry, Johns Hopkins University, 2009
Postdoctoral Fellow, California Institute of Technology, 2009-2011
Research
For more information visit: http://www.uncg.edu/che/faculty/petersen/index.html
The Petersen research group is focused on solving important synthetic problems using basic principles of organic chemistry. In particular we are interested in the development of new methods for the asymmetric synthesis of biologically important molecules and in the design and synthesis of new drug targets.
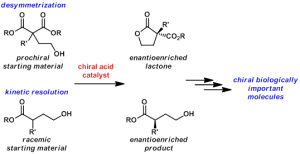 Many biologically important compounds contain a chiral center whose absolute configuration is critical for activity. Compounds found in nature that are identified with prospective utility in human health frequently exist as a complex mixture and are produced in such small quantities that they cannot be fully vetted for their potential. The exploration and future use of these precious materials often is contingent on the ability of a synthetic chemist to prepare them in an efficient and selective manner. Key to success of a laboratory synthesis of an enantioenriched complex molecule is the availability of simple enantioenriched building blocks. The ideal building block would have handles that are readily transformed into a variety of functionalities that can be incorporated into the biologically important molecule. A major focus of our research laboratory is the development of asymmetric methodologies for the synthesis of versatile enantioenriched building blocks.
Many biologically important compounds contain a chiral center whose absolute configuration is critical for activity. Compounds found in nature that are identified with prospective utility in human health frequently exist as a complex mixture and are produced in such small quantities that they cannot be fully vetted for their potential. The exploration and future use of these precious materials often is contingent on the ability of a synthetic chemist to prepare them in an efficient and selective manner. Key to success of a laboratory synthesis of an enantioenriched complex molecule is the availability of simple enantioenriched building blocks. The ideal building block would have handles that are readily transformed into a variety of functionalities that can be incorporated into the biologically important molecule. A major focus of our research laboratory is the development of asymmetric methodologies for the synthesis of versatile enantioenriched building blocks.
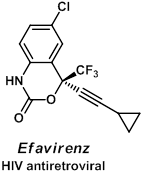 In recent years, fluorine containing molecules have become important in the pharmaceutical and agrochemical industries, and in particular, the trifluoromethyl group appears in many biologically active compounds such as the HIV antiretroviral drug Efavirenz. The increased lipophilicity and metabolic stability of the CF3 group often accounts for an improved activity profile. Unfortunately, the regio- and stereoselective incorporation of the trifluoromethyl group into organic molecules remains a challenge for chemists. Research toward the selective incorporation of this important functional group is another focus in our group.
In recent years, fluorine containing molecules have become important in the pharmaceutical and agrochemical industries, and in particular, the trifluoromethyl group appears in many biologically active compounds such as the HIV antiretroviral drug Efavirenz. The increased lipophilicity and metabolic stability of the CF3 group often accounts for an improved activity profile. Unfortunately, the regio- and stereoselective incorporation of the trifluoromethyl group into organic molecules remains a challenge for chemists. Research toward the selective incorporation of this important functional group is another focus in our group.
Despite recent advances in drug therapies for diseases such as cancer and malaria there is an essential need for ever more innovative and effective small molecule therapeutics. The Petersen group is active in the design and synthesis of new scaffolds with antimalarial or anticancer properties, in particular novel peroxides and diphenylpropynones.
Representative Publications
- Petersen, K. S.; Stoltz, B. M. Palladium-catalyzed, asymmetric Baeyer–Villiger oxidation of prochiral cyclobutanones with PHOX ligands. Tetrahedron 2011, 67, 4352–4357.http://www.sciencedirect.com/science/article/pii/S0040402011005539
- Posner, G. H.; Chang, W.; Hess, L.; Woodard, L.; Sinishtaj, S.; Usera, A. R.; Maio, W.; Rosenthal, A. S.; Kalinda, A. S.; Petersen, K. S.; Stohler, R.; Chollet, J.; Santo-Tomas, J.; Snyder, C.; Rottmann, M.; Wittlin, S.; Brun, R.; Shapiro, T. A. Malaria-infected mice are cured by oral administration of new artemisinin derivatives. J. Med. Chem. 2008, 51, 1035–1042. http://pubs.acs.org/doi/abs/10.1021/jm701168h
- Petersen, K. S.; Posner, G. H. Asymmetric, organocatalytic, 3-step synthesis of γ-hydroxy-(E)-α,β-unsaturated sulfones and esters. Org. Lett. 2008, 10, 4685–4687. http://pubs.acs.org/doi/abs/10.1021/ol8020513
- Posner, G. H.; Suh, B. C.; Petersen, K. S.; Dolan, P.; Kensler, T. W.; Koh, J. T.; Peleg, S. Difluoromethyl analogs of the natural hormone 1α,25-dihydroxyvitamin D3: design, synthesis, and preliminary biological evaluation. J. Steroid Biochem. Mol. Biol. 2007, 103, 213–221. http://www.sciencedirect.com/science/article/pii/S0960076006003724
- Petersen, K. S.; Dolan, P. M.; Kensler, T. W.; Peleg, S.; Posner, G. H. Low-calcemic, highly antiproliferative, 23-oxa ether analogs of the natural hormone 1α,25-dihydroxyvitamin D3: design, synthesis, and preliminary biological evaluation. J. Med. Chem. 2007, 50, 5824–5832. http://pubs.acs.org/doi/abs/10.1021/jm070882d
- Peleg, S.; Petersen, K. S.; Suh, B. C.; Dolan, P.; Agoston, E. S.; Kensler, T. W.; Posner, G. H. Low-calcemic, highly antiproliferative, 1-difluoromethyl hybrid analogs of the natural hormone 1α,25-dihydroxy D3: design, synthesis, and preliminary biological evaluation. J. Med. Chem. 2006, 49, 7513–7517.http://pubs.acs.org/doi/abs/10.1021/jm0609925
- Thibout, E.; Arnault, I.; Auger, J.; Petersen, K. S.; Oliver, J. E. Characterization of a behaviorally active, gender-specific volatile compound from the male asparagus fly. J. Chem. Ecol. 2005, 31, 893–909.http://www.springerlink.com/content/m8256035522rp387/
- Copper, L. D.; Doss, R. P.; Price, R.; Peterson, K.; Oliver, J. E. Application of Bruchin B to pea pods results in the up-regulation of CYP93C18, a putative isoflavone synthase gene, and an increase in the level of pisatin, an isoflavone phytoalexin. J. Exp. Bot. 2005, 56, 1229–1237. http://jxb.oxfordjournals.org/content/56/414/1229.abstract





