Petersen Research Group
The Petersen research group is focused on solving important synthetic problems using basic principles of organic chemistry. In particular we are interested in the development of new methods for the asymmetric synthesis of biologically important molecules and in the design and synthesis of new drug targets.
Research
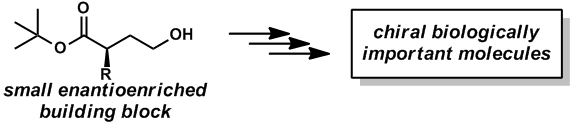
Many biologically important compounds contain a chiral center who’s absolute configuration is critical for activity. Compounds found in nature that are identified with prospective utility in human health frequently exist as a complex mixture and are produced in such small quantities that they cannot be fully vetted for their potential. The exploration and future use of these precious materials often is contingent on the ability of a synthetic chemist to prepare them in an efficient and selective manner. Key to success of a laboratory synthesis of an enantioenriched complex molecule is the availability of simple enantioenriched building blocks. The ideal building block would have handles that are readily transformed into a variety of functionalities that can be incorporated into the biologically important molecule. A major focus of our research laboratory is the development of asymmetric methodologies for the synthesis of versatile enantioenriched building blocks.
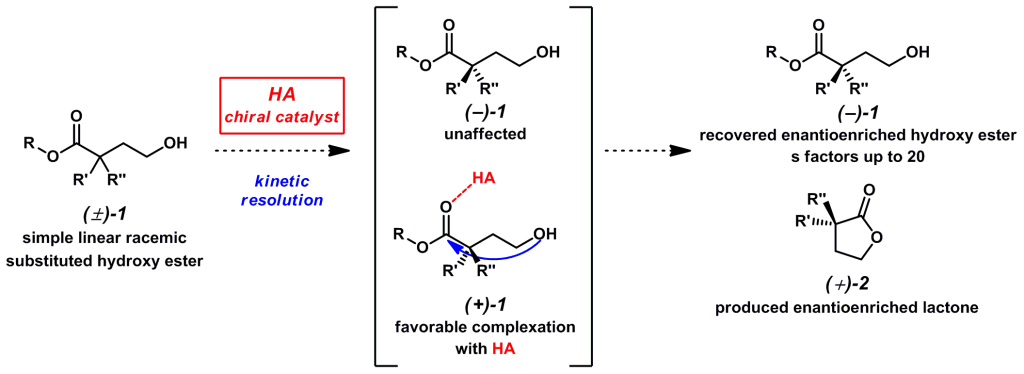
Current efforts have been directed toward an efficient synthesis of enantioenriched a-substituted hydroxy esters via a kinetic resolution event is described. Bulky racemic esters in the presence of a chiral Brønsted acid selectively lactonize to yield a recoverable en-antioenriched hydroxy ester and lactone. These esters are highly versatile building blocks that can readily be converted to synthetically useful materials.
Publications
Qabaja, G.; Benavides, A. R.; Shubin, L.; Petersen, K. S. Facile Synthesis of Versatile Enantiosenriched α-Substituted Hydroxy Esters through a Brønsted Acid Catalyzed Kinetic Resolution Asymmetric Synthesis of Hydroxy Esters with Multiple Stereocenters via a Chiral Phosphoric Acid Catalyzed Kinetic Resolution. J. Org. Chem. http://pubs.acs.org/doi/abs/10.1021/jo5022019
Qabaja, G.; Wilent, J. E.; Benavides, A. R.; Bullard, G. E.; Petersen, K. S. Facile Synthesis of Versatile Enantiosenriched α-Substituted Hydroxy Esters through a Brønsted Acid Catalyzed Kinetic Resolution.Org. Lett. 2013, 15, 1266–1269. http://pubs.acs.org/doi/abs/10.1021/ol400207t
Wilent, J. E.; Petersen, K. S. Enantioselective Desymmetrization of Diesters. J. Org. Chem. 2014, 79, 2303–2307. http://pubs.acs.org/doi/abs/10.1021/jo402853v

In recent years, fluorine containing molecules have become important in the pharmaceutical and agrochemical industries, and in particular, the trifluoromethyl group appears in many biologically active compounds such as the HIV antiretroviral drug Efavirenz. The increased lipophilicity and metabolic stability of the CF3 group often accounts for an improved activity profile. Unfortunately, the regio- and stereoselective incorporation of the trifluoromethyl group into organic molecules remains a challenge for chemists. Research toward the selective incorporation of this important functional group is another focus in our group.
Publications
Sakavuyi, K., Petersen, K. S. Nucleophilic trifluoromethylation of conjugate acceptors via phenyl trifluoromethyl sulfone. Tetrahedron Lett. (2013), http://dx.doi.org/10.1016/j.tetlet.2013.08.132

Despite recent advances in drug therapies for diseases such as cancer and malaria there is an essential need for ever more innovative and effective small molecule therapeutics. The Petersen group is active in the design and synthesis of new scaffolds with antimalarial or anticancer properties, in particular novel peroxides and diphenylpropynones.
Publications coming soon!

