Hematian Research Group
The Hematian Research Group is interested in biological and energy sciences from an inorganic chemistry perspective. We combine a variety of research tools to address fundamental challenges related to biochemical and chemical catalysis, sustainability, and renewable energy. Our research is targeted towards manipulation of a broad range of redox processes that take place within homogeneous photocatalysts, protein scaffolds, and electrochemical systems.
Metalloproteins have many different functions in cells, such as electron transfer, O2-transport, O2-reduction (oxidase activity), biological substrate oxygenation (oxygenase activity) or reduction of a variety of other biological substrates such as nitrogen oxides (NOx), molecular nitrogen (N2), carbon dioxide, hydrogen peroxide, sulfate, etc. Central to all of these processes is the presence of the unique metal cofactors within protein frameworks that allows metalloenzymes to perform functions such as redox reactions through optimizing the flow of protons and electrons between their various substrates.
Using nature as inspiration, we seek to understand the fundamental mechanisms underlying the role of metal ion cofactors in enzymes and developing artificial metalloenzyme or bio-inspired synthetic systems for applications in catalysis and beyond. We draw on expertise from a variety of areas including bioinorganic chemistry, inorganic synthesis, chemical biology, materials chemistry, and electrochemistry. Our group started off in August of 2018 and our work covers many different aspects of redox chemistry.
Research
Copper Enzymes
Enzymatic Function, Substrate Specificity, and Mechanistic Studies
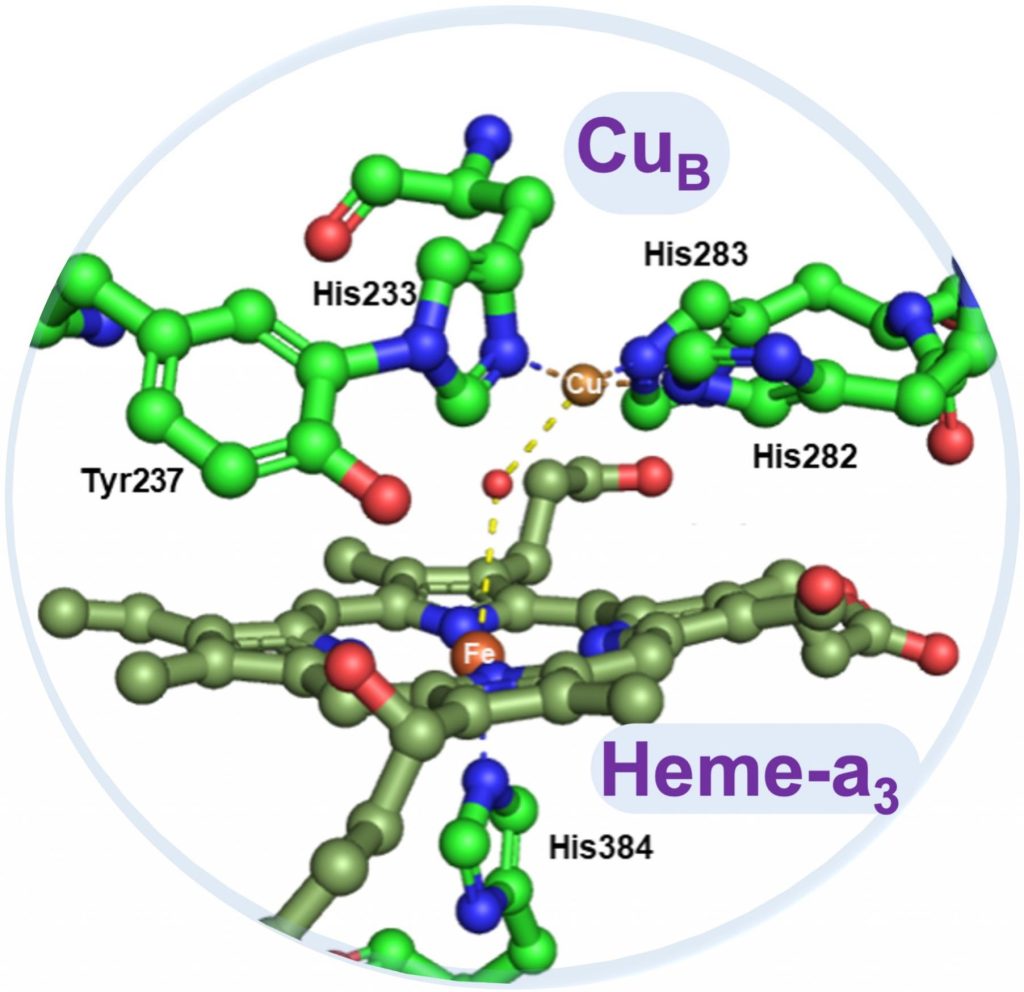
Aerobic life on earth harnesses the oxidizing power of molecular oxygen (O2) through a diverse range of enzyme cofactors and employs that high oxidation potential to mediate numerous oxidative transformations during metabolic functions. Many of these cofactors are coupled binuclear sites consisting of two metal centers such as copper and iron in close proximity. Our team focuses on fundamental research in the field of bioinorganic chemistry and aims to address several intriguing questions about the potential roles of redox aromatic active amino acids such as tyrosine and tryptophan chains in the enzyme mechanism and function.
We work on three different classes of O2-utilizing copper enzymes including cytochrome c oxidase (CcO) which reduces O2 to water, multicopper oxidase (MCO) which couples that reaction to four one-electron oxidations of the substrates, and a new class of copper enzymes called BURP domain cyclases which catalyze 2-electron oxidative macrocyclization of the Tyr/Trp chains in peptide substrates. In all these enzymes, O2 serves as a terminal electron acceptor.
In all three classes, appropriately tuned and positioned Tyr/Trp chains play different roles either as an integral part of the active site (i.e., CcO) or they may be assigned a mediatory role to provide an alternative path for oxidation of the more challenging substrates (i.e., MCO). They may even act as the direct substrate for the active site (i.e., Cu-dependent BURP domain cyclases). In all cases, despite the diverse use of these chains, their main function is to delicately supply the electron/proton needed for the O2 reduction. How are these residues designed/optimized for a particular function? What are the similarities and differences between these enzymes? Our studies aim to reveal the potential role of these Tyr/Trp chains play in enzymatic function and mechanism and address some of the questions about copper biochemistry and aerobic metabolism.
Mild Metal-Catalyzed C–H Activation
Alternative Synthesis for Oxoiron(IV) Species
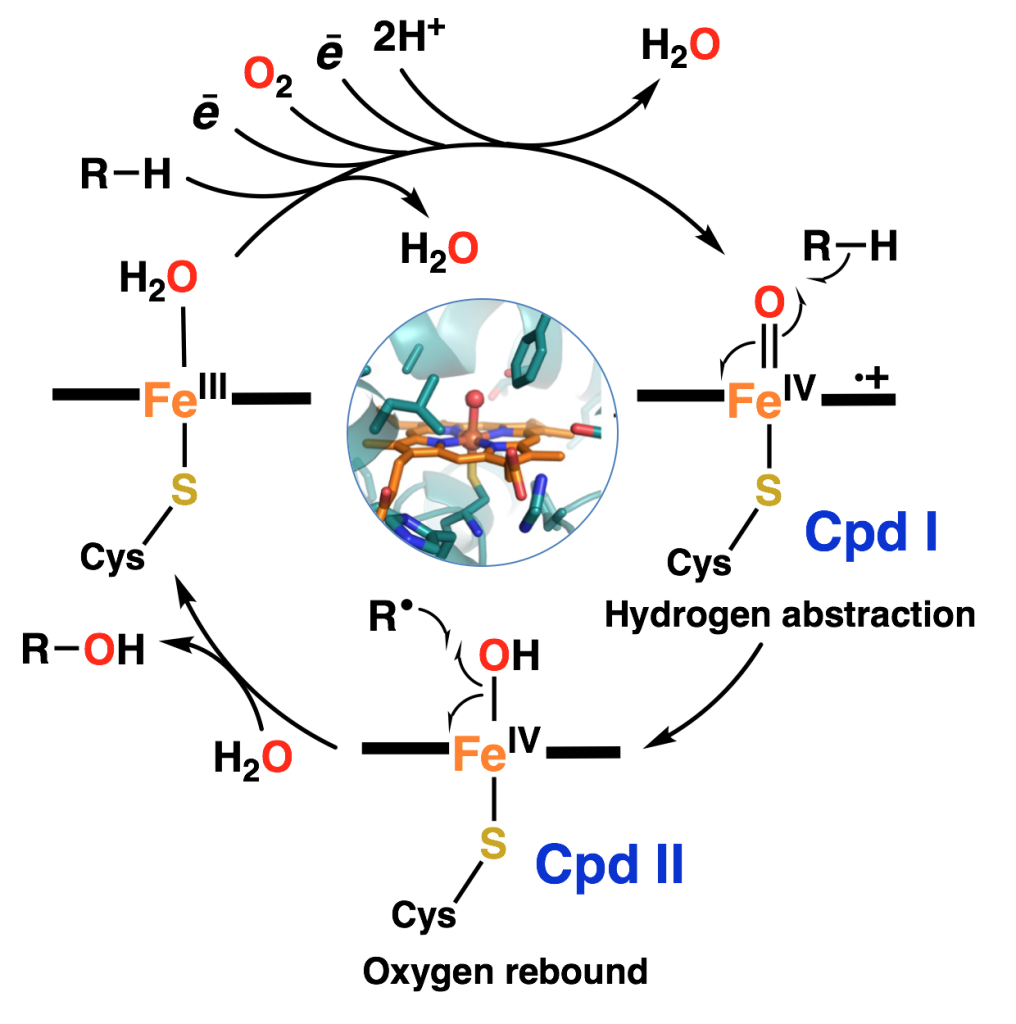
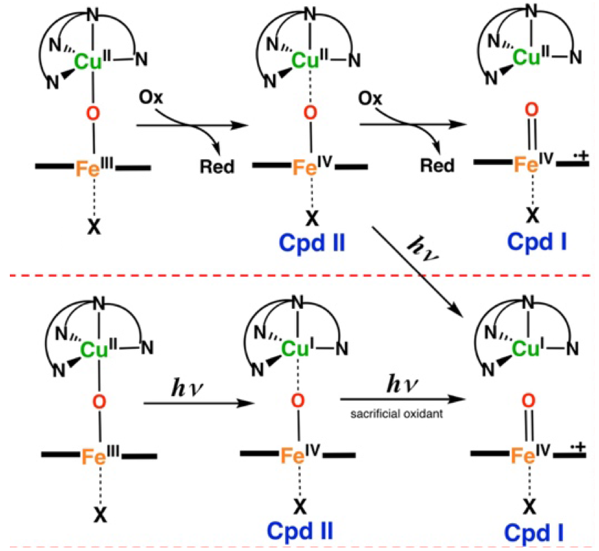
High-valent iron-oxo porphyrin species (compound I (Cpd I) and compound II (Cpd II)) are invoked as key oxidizing intermediates in the catalytic cycles of a broad spectrum of heme enzymes and are important reactive species in catalysis. In heme enzymes, these species are formed from dioxygen, whereas high-energy oxidants are typically required to prepare their synthetic analogues.
Our new approach for generation and study of synthetic Cpd I and II analogues is through the one or two electron oxidation of μ-oxo heme/Cu complexes, with mild oxidants. These synthetic heme-copper oxidase model complexes, possess many interesting structural and reactivity properties that make them promising precursors for the generation of synthetic high-valent iron-oxo porphyrins. The primary objective of this project is to tune the electron density residing on the μ-oxo moiety through modification of the copper coordination environment, axial ligands, and substituents on the porphyrin ring, thereby allowing for more accessible high-valent iron oxo species for synthetic applications and fundamental characterization. We also use laser flash photolysis to prepare synthetic Cpd I or II analogues from the μ-oxo heme/Cu compounds.
Enhancing Carbon Dioxide Aqueous Electrolysis
Tuning and Optimization of Electrocatalysts and Electrolyte Composition
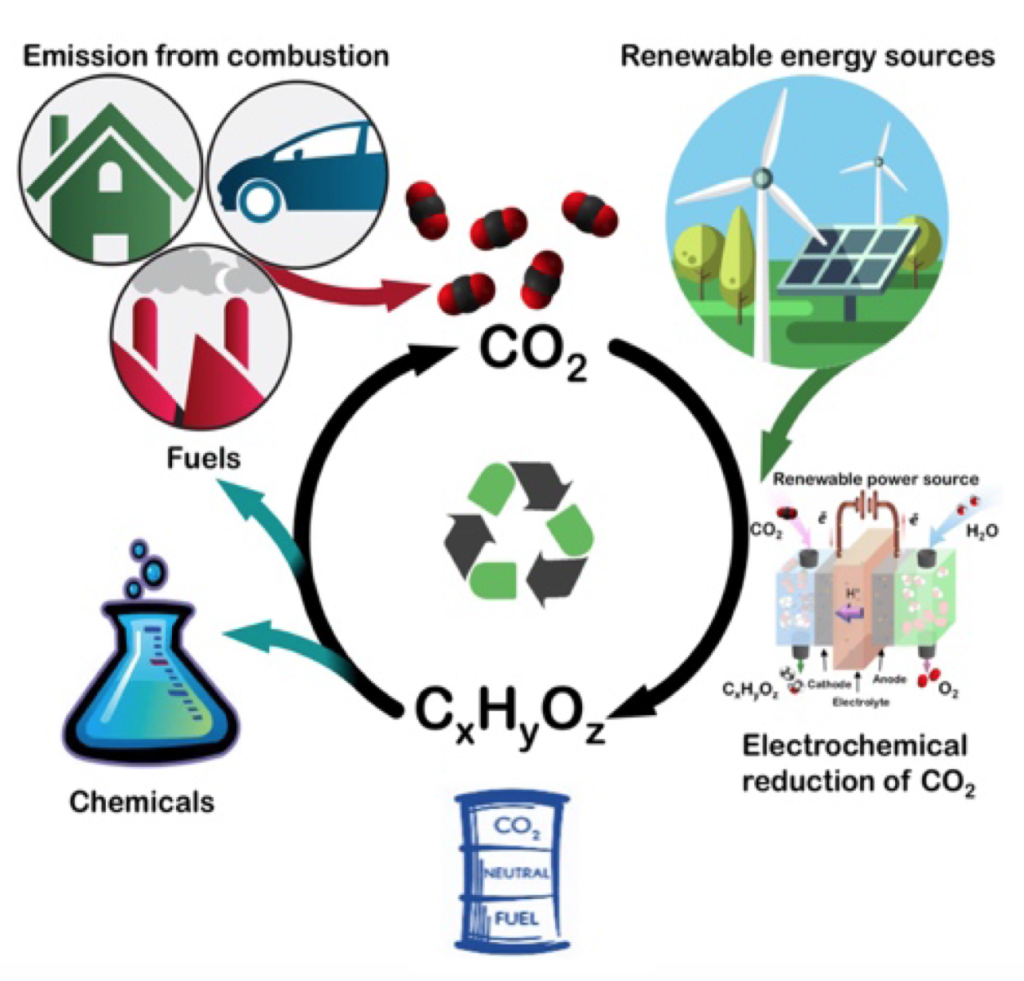
Conversion of the greenhouse gas carbon dioxide (CO2) to value-added products is an important challenge for sustainable energy research. The use of coordination and organometallic complexes as precursors for the synthesis of multimetallic/metal oxide nanoparticles is a promising approach that can be used to obtain a broad class of heterogeneous catalysts for such transformations. The multifunctional catalytic sites available at the interface of these multimetallic electrocatalysts can promote the stability of key intermediates through synergistic interactions between two metals, thus facilitating CO2 reduction. Additionally, an often overlooked aspect of electrocatalytic CO2 reduction is the electrolyte. Thus, we investigate the potential role of unconventional electrolytes in electrochemical reduction of CO2.
Around The Lab










