Publications
2023
| 40 |
“Arene Additions”Abraham Ustoyev, Mitchell P. Croatt Nature Chemistry 2023, online. https://www.nature.com/articles/s41557-023-01190-5 (Highlight of of a wonderful paper by Fokin and coworkers.)
|
| 39 |
“Thienopyrimidine Derivatives as GPR55 Receptor Antagonists: Insight into Structure–Activity Relationship”Laura Figuerola-Asencio, Paula Morales, Pingwei Zhao, Dow P. Hurst, Sommayah S. Sayed, Katsuya L. Colón, María Gómez-Cañas, Javier Fernández-Ruiz, Mitchell P. Croatt, Patricia H. Reggio, Mary E. Abood, and Nadine Jagerovic ACS Medicinal Chemistry Letters 2023, 14, 18-25. https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00325
|
2022
| 38 |
“Semisynthesis of Hypothemycin Analogues Targeting the C8-C9 Diol”Zeinab Y. Al Subeh, Tian Li, Abraham Ustoyev, Jennifer C. Obike, Philip M. West, Manead Khin, Joanna E. Burdette, Cedric J. Pearce, Nicholas H. Oberlies, and Mitchell P. Croatt Journal of Natural Products 2022, 85, 2018-2025. https://pubs.acs.org/doi/10.1021/acs.jnatprod.2c00434
|
| 37 |
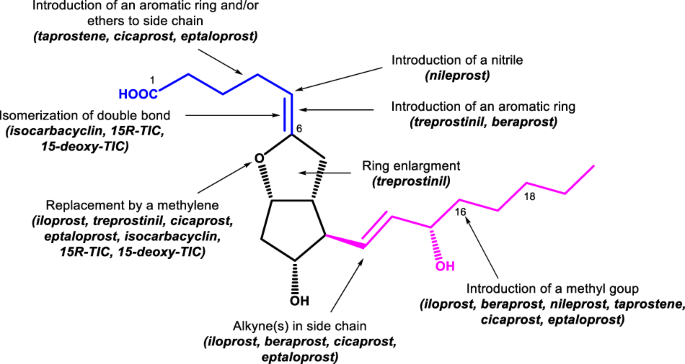
“Prostacyclin (PGI2) scaffolds in medicinal chemistry: current and emerging drugs”Ghina’a I. Abu Deiab and Mitchell P. Croatt Medicinal Chemistry Research 2022, 31, 1241-1251. https://link.springer.com/article/10.1007/s00044-022-02914-x
|
| 36 |
“AlCl3-catalyzed regioselective intermolecular α or γ mono- or α,γ bis-hydroalkoxylation of allenamides with alcohols”Khyarul Alam, Tian Li, I. F. Dempsey Hyatt, and Mitchell P. Croatt Organic & Biomolecular Chemistry 2022, 20, 4719-4723. https://pubs.rsc.org/en/content/articlelanding/2022/ob/d2ob00844k
|
| 35 |
“Rapid and Facile Synthesis of Isomaleimides: Dehydration of Maleamic Acids using Methanesulfonyl Chloride”Khyarul Alam, Elvis C. McFee, and Mitchell P. Croatt Synthesis 2022, 54, 3085-3092. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0041-1737414
|
2021
| 34 |
“Media and strain studies for the scaled production of cis-enone resorcylic acid lactones as feedstocks for semisynthesis”Zeinab Y. Al Subeh, Huzefa A. Raja, Jennifer C. Obike, Cedric J. Pearce, Mitchell P. Croatt, and Nicholas H. Oberlies The Journal of Antibiotics 2021, 74, 496-507. https://www.nature.com/articles/s41429-021-00432-3
|
2019
| 33 |
“Metal‐Catalyzed Dehydration of Primary Amides to Nitriles”Mohammed H. Al-Huniti and Mitchell P. Croatt Asian Journal of Organic Chemistry 2019, 8, 1791-1799. https://onlinelibrary.wiley.com/doi/10.1002/ajoc.201900343
|
| 32 |
“Synthetic Approaches to Isocarbacyclin and Analogues as Potential Neuroprotective Agents Against Ischemic Stroke”Ghina’a I. Abu Deiab and Mitchell P. Croatt Bioorganic & Medicinal Chemistry 2019, 27, 338-342. https://www.sciencedirect.com/science/article/pii/S0968089618318972?via%3Dihub
|
2018
| 31 |
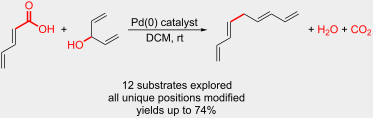
“Palladium-Catalyzed Chemoselective Protodecarboxylation of Polyenoic Acids”Mohammed H. Al-Huniti, Mark A. Perez, Matthew K. Garr, and Mitchell P. Croatt Organic Letters 2018, 23, 7375-7379. https://pubs.acs.org/doi/10.1021/acs.orglett.8b03016
|
| 30 |
“DFT Mechanistic Investigation of an Enantioselective Tsuji-Trost Allylation Reaction”Kate E. McPherson, Mitchell P. Croatt, Andrew T. Morehead, Jr., and Andrew L. Sargent Organometallics 2018, 37, 3791-3802. https://pubs.acs.org/doi/full/10.1021/acs.organomet.8b00507
|
| 29 |
“Development and Utilization of a Palladium-Catalyzed Dehydration of Primary Amides To Form Nitriles”Mohammed H. Al-Huniti, José Rivera-Chávez, Katsuya L. Colón, Jarrod L. Stanley, Joanna E. Burdette, Cedric J. Pearce, Nicholas H. Oberlies, and Mitchell P. Croatt Organic Letters 2018, 20, 6046-6050. https://pubs.acs.org/doi/10.1021/acs.orglett.8b02422
|
2017
| 28 |
“Hypervalent Iodonium Alkynyl Triflate Generated Phenylcyanocarbene and Its Reactivity with Aromatic Systems”Mohammed H. Al-Huniti, Zachary B. Sullivan, Jarrod L. Stanley, James A. Carson, I. F. Dempsey Hyatt, A. Christina Hairston, and Mitchell P. Croatt Journal of Organic Chemistry 2017, 82, 11772-11780. http://pubs.acs.org/doi/10.1021/acs.joc.7b01608 (Special Issue on Hypervalent Iodine)
|
| 27 |
“Chemoselective fluorination and chemoinformatic analysis of griseofulvin: natural vs fluorinated fungal metabolites”Noemi D. Paguigan, Mohammed Al-Huniti, Huzefa A. Raja, Austin Czarnecki, Joanna E. Burdette, Mariana González-Medina, José L. Medina-Franco, Stephen J. Polyak, Cedric J. Pearce, Mitchell P. Croatt, Nicholas H. Oberlies Bioorganic & Medicinal Chemistry 2017, 25, 5238-5246. http://www.sciencedirect.com/science/article/pii/S0968089617306521
|
| 26 |
“Design, synthesis and biological evaluation of GPR55 agonists”Lara Fakhouri, Christopher D. Cook, Mohammed H. Al-Huniti, Linda M. Console-Bram, Dow P. Hurst, Michael B.S. Spano, Daniel J. Nasrallah, Marc G. Caron, Larry S. Barak, Patricia H. Reggio, Mary E. Abood, Mitchell P. Croatt Bioorganic & Medicinal Chemistry 2017, 25, 4355-4367. http://www.sciencedirect.com/science/article/pii/S0968089617306521
|
| 25 |
“Decarboxylative and dehydrative coupling of dienoic acids and pentadienyl alcohols to form 1,3,6,8-tetraenes”Ghina’a I. Abu Deiab, Mohammed H. Al-Huniti, I. F. Dempsey Hyatt, Emma E. Nagy, Kristen E. Gettys, Sommayah S. Sayed, Christine M. Joliat, Paige E. Daniel, Rupa M. Vummalaneni, Andrew T. Morehead Jr, Andrew L. Sargent and Mitchell P. Croatt Beilstein Journal of Organic Chemistry 2017, 13, 384-392. https://www.beilstein-journals.org/bjoc/articles/13/41
|
| 24 |
“Structure-Activity Relationships of Benzothiazole GPR35 Antagonists”Manahil M. Abdalhameed, Pingwei Zhao, Dow P. Hurst, Patricia H. Reggio, Mary E. Abood, and Mitchell P. Croatt Bioorganic & Medicinal Chemistry Letters 2017, 27, 612-615. http://www.sciencedirect.com/science/article/pii/S0960894X16312793
|
| 23 |
“Step-Economical Synthesis of Clinprost and Analogues Utilizing a Novel Decarboxylation Reaction”Ghina’a I. Abu-Deiab and Mitchell P. Croatt In Strategies and Tactics in Organic Synthesis Harmata, M. Ed.; Academic Press, 2017, Vol. 12, pp 95-117; http://www.sciencedirect.com/science/article/pii/B9780081007563000030
|
2016
| 22 |
“Design, Synthesis, and Analysis of Antagonists of GPR55: Piperidine-Substituted 1,3,4-oxadiazol-2-ones”Maria Elena Meza-Avina, Mary A. Lingerfelt, Linda Console-Bram, Thomas F. Gamage, Haleli Sharir, Kristen E. Gettys, Dow P. Hurst, Evangelia Kotsikorou, Derek M Shore, Marc G. Caron, Narasinga Rao, Larry S. Barak, Mary E. Abood, Patricia H. Reggio, and Mitchell P Croatt Bioorganic & Medicinal Chemistry Letters 2016, 26, 1827-1830. http://www.sciencedirect.com/science/article/pii/S0960894X16301421
|
2015
| 21 |
“Spiroscytalin, a New Tetramic Acid and Other Metabolites of Mixed Biogenesis from Scytalidium cuboideum“Arlene A. Sy-Cordero, Mario Figueroa, Huzefa A. Raja, Maria Elena Meza-Avina, Mitchell P. Croatt, Audrey R. Adcock, David J. Kroll, Mansukh C. Wani, Cedric J. Pearce, and Nicholas H. Oberlies Tetrahedron 2015, 71, 8899-8904. http://www.sciencedirect.com/science/article/pii/S0040402015300971
|
| 20 |
“Isolation, semisynthesis, covalent docking and transforming growth factor beta-activated kinase 1 (TAK1)-inhibitory activities of (5Z)-7-oxozeaenol analogues”Lara Fakhouri, Tamam El-Elimat, Dow P. Hurst, Patricia H. Reggio, Cedric J. Pearce, Nicholas H. Oberlies, and Mitchell P. Croatt Bioorganic & Medicinal Chemistry 2015, 23, 6993-6999. http://www.sciencedirect.com/science/article/pii/S0968089615300626
|
| 19 |
“Formation and in situ reactions of hypervalent iodonium alkynyl triflates to form cyanocarbenes”I. F. Dempsey Hyatt, Daniel J. Nasrallah, Michael A. Maxwell, A. Christina F. Hairston, Manahil M. Abdalhameed, and Mitchell P. Croatt Chemical Communications 2015, 51, 5287-5289. http://pubs.rsc.org/en/content/articlelanding/2015/cc/c4cc08676g/unauth#!divRelatedContent
|
2014
| 18 |
“Rhodium(I)-Catalyzed [2+2+2+2] Cycloaddition of Diynes to Form Cyclooctatetraenes”Daniel J. Nasrallah and Mitchell P Croatt European Journal of Organic Chemistry 2014, 2014, 3767-3772. http://dx.doi.org/10.1002/ejoc.201402109
|
2013
| 17 |
“Identification of the GPR55 Antagonist Binding Site Using a Novel Set of High-Potency GPR55 Selective Ligands”Evangelia Kotsikorou, Haleli Sharir, Derek M Shore, Dow P. Hurst, Diane L. Lynch, Karla E. Madrigal, Susanne Heynen-Genel, Loribelle B. Milan, Thomas D.Y. Chung, Herbert H. Seltzman, Yushi Bai, Marc G. Caron, Lawrence S. Barak, Mitchell P Croatt, Mary E Abood, and Patricia H. Reggio Biochemistry 2013, 52, 9456-9469. http://pubs.acs.org/doi/abs/10.1021/bi4008885
|
| 16 |
“Synthesis of Hypervalent Iodonium Alkynyl Triflates for the Application of Generating Cyanocarbenes”I. F. Dempsey Hyatt, Daniel J. Nasrallah, Mitchell P. Croatt Journal of Visualized Experiments 2013, 79, e50886. doi:10.3791/50866. http://www.jove.com/video/50886/synthesis-hypervalent-iodonium-alkynyl-triflates-for-application
|
| 15 |
“Mechanistic Study of the Biomimetic Synthesis of Flavonolignan Diastereoisomers in Milk Thistle”Hanan S. Althagafy, Maria Elena Meza-Aviña, Nicholas H. Oberlies, Mitchell P. Croatt Journal of Organic Chemistry 2013, 78, 7594-7600. http://pubs.acs.org/doi/abs/10.1021/jo4011377
|
| 14 |
“Exploring the reactivity of 1,5-disubstituted sulfonyl-triazoles: Thermolysis and Rh(II)-catalyzed synthesis of α-sulfonyl nitriles”Maria Elena Meza-Aviña, Mudita Kishor Patel, and Mitchell P. Croatt Tetrahedron 2013, 69, 7840-7846. http://www.sciencedirect.com/science/article/pii/S0040402013007837 (Special Issue in Honor of Professor Wender)
|
| 13 |
“Semisynthesis, cytotoxicity, antiviral activity, and drug interaction liability of 7-O-methylated analogues of flavonolignans from milk thistle”Hanan S. Althagafy, Tyler N. Graf, Arlene A. Sy-Cordero, Brandon T. Gufford, Mary F. Paine, Jessica Wagoner, Stephen J. Polyak, Mitchell P. Croatt, Nicholas H. Oberlies Bioorganic & Medicinal Chemistry 2013, 21, 3919-3926. http://www.sciencedirect.com/science/article/pii/S0968089613003349
|
| 12 |
“Sequential Pd(0)-, Rh(I)-, and Ru(II)-catalyzed Reactions in a Nine-step Synthesis of Clinprost”Emma E. Nagy, I. F. Dempsey Hyatt, Kristen E. Gettys, Shawn T. Yeazell, Stephen K. Frempong Jr., and Mitchell P. Croatt Organic Letters 2013, 15, 586-589. http://pubs.acs.org/doi/abs/10.1021/ol303402e (Highlighted in SYNFACTS 2013, 9, 470)
|
2012
| 11 |
“Alkynes and Azides: Not Just for Click Reactions”I. F. Dempsey Hyatt, Maria Elena Meza-Aviña, and Mitchell P. Croatt Synlett 2012, 23, 2869-2874. https://www.thieme-connect.com/ejournals/abstract/10.1055/s-0032-1317545 (Invited SYNPACTS article)
|
| 10 |
“Reactions of Hypervalent Iodonium-Alkynyl Triflates with Azides: New Mechanistic Approach for the Generation of Cyanocarbenes”I. F. Dempsey Hyatt, Mitchell P. Croatt http://onlinelibrary.wiley.com/doi/10.1002/anie.201203062/abstract (Featured with the Front Cover Graphic of that issue of Angew. Chem., Int. Ed. and Highlighted in Angew. Chem. Int. Ed. 2012, 51, 12169-12171.)
|
2011
| 9 |
“Selective Formation of 1,5-Substituted Sulfonyl Triazoles Using Acetylides and Sulfonyl Azides”Maria Elena Meza-Aviña, Mudita Kishor Patel, Cylivia B. Lee, Thomas J. Dietz, Mitchell P. Croatt Organic Letters 2011, 13, 2984-2987; http://pubs.acs.org/doi/full/10.1021/ol200696q (Highlighted in SYNFACTS 2011, 9, 1005)
|
| 8 |
“Probing the Role of the Mycosamine C2′-OH on the Activity of Amphotericin B.”Mitchell P. Croatt, Erick M. Carreira Organic Letters 2011, 13, 1390-1393; |
2010
| 7 |
“The Diene Effect: The Design, Development, and Mechanistic Investigation of Metal-Catalyzed Diene-yne, Diene-ene, and Diene-allene [2+2+1] Cycloaddition Reactions”Mitchell P. Croatt, Paul A. Wender. European Journal of Organic Chemistry 2010, 2010, 19-32; http://onlinelibrary.wiley.com/doi/10.1002/ejoc.200900929/abstract |
2009
| 6 |
“Rhodium(I)-Catalyzed [2+2], [2+2+2], and [2+2+2+2] Cycloadditions of Dienes or Alkynes with a Bis-ene”Paul A. Wender, Mitchell P. Croatt, Björn Kühn Organometallics 2009, 28, 5841-5844; |
2007
| 5 |
“Higher Order Cycloadditions”Paul A. Wender, Mitchell P. Croatt, Nicole M. Deschamps In Comprehensive Organometallic Chemistry III; Crabtree, R. H.; Mingos, D. M. P. Eds.; Elsevier: Oxford, 2007; Vol. 10, pp 603-648; http://www.elsevier.com/wps/find/bookdescription.cws_home/721739/description |
2006
| 4 |
“Metal-Catalyzed [2+2+1] Cycloadditions of 1,3-Dienes, Allenes, and CO”Paul A. Wender, Mitchell P. Croatt, Nicole M. Deschamps Angewandte Chemie International Edition 2006, 45, 2459-2462; http://onlinelibrary.wiley.com/doi/10.1002/anie.200600300/abstract |
| 3 |
“Carbonyl(chloro)bis(triphenylphosphine)rhodium(I)”Mitchell P. Croatt, Travis J. Williams, Paul A. Wender Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, Ltd., 2006, online; http://onlinelibrary.wiley.com/o/eros/articles/rc021/frame.html |
| 2 |
“New reactions and step economy: the total synthesis of (±)-salsolene oxide based on the type II transition metal-catalyzed intramolecular [4+4] cycloaddition”Paul A. Wender, Mitchell P. Croatt, Bernhard Witulski Tetrahedron 2006, 62, 7505-7511; http://www.sciencedirect.com/science/article/pii/S0040402006006740 |
2004
| 1 |
“Rhodium(I)-Catalyzed [2+2+1] Cycloadditions of 1,3-Dienes, Alkenes, and CO”Paul A. Wender, Mitchell P. Croatt, Nicole M. Deschamps Journal of the American Chemical Society 2004, 126, 5948-5949; |