People
Terence Nile

Field: Inorganic Chemistry
Room: 405 Sullivan Science Building
Phone: 336.334.5139
Email: terry_nile@uncg.edu
Education
B.Sc (1966)
M.Sc (1970)
D. Phil. (1975) University of Sussex
Research
Over the past few years, the focus of our research has been the synthesis of novel substituted cyclopentadienes and their transition metal complexes. Our goal has been to increase the number and variety of substituted cyclopentadienes available, and to evaluate their steric and electronic properties as ligands. These results will allow rational choices to be made in the design of cyclopentadienyl metal complexes for use in specific catalytic and stoichiometric reactions.
A. Complexes of tetrahydroindenes
Complexes of 4,5,6,7-tetrahydroindene have been the focus of recent great interest due to the catalytic activity of their zirconium compounds. 1 These complexes have been synthesized by the hydrogenation of the six-membered aromatic ring of the corresponding complexed indenyl ligands. 2 This route limits the synthesis of an extensive range of such complexes, especially those which contain other moieties that are sensitive to hydrogenation.
We have devised a straight-forward, two-step synthesis of 4,5,6,7-tetrahydroindene, Cp oH, 1, and its methylated congeners 1-methyl-, 1,3-dimethyl- and 1,2,3-trimethyl-4,5,6,7-tetrahydroindene, 2-4, Cp 1H, Cp 2H and Cp 3H, respectively 3. These are synthesized via the intermediate bicyclic cyclopentenones which can be easily isolated in 20-60% using the Dev 4 modification of the Nazarov cyclization reaction. Nucleophilic addition of hydride (from LiAIH 4) or methyl (from methyllithium) anions give allylic alcohols, which are subsequently dehydrated, in situ, using acid catalysis. This addition-dehydration process yields the 4,5,6,7-tetrahydroindenes, 1-4, in 25-50%.
We now propose to synthesize ligands and complexes that should prove very active catalysts for the polymerization of alkenes. We will synthesize the dimethylsilyl bridged bis(tetrahydroindenyl)ligands, Cp x 2SiMe 2, by the reaction of the anions derived from 1–4with dimethyldichlorosilane. These cyclopentadienes will be converted, via their dianions, to metallocene dihalides, equation 1.
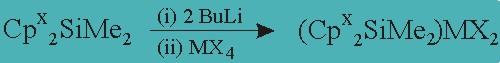
x = 0, 1, 2: M = Ti, Zr, Hf: X = Cl, Br, I
The catalytic activity of these complexes, in conjunction with an activator such as methylalumoxane, MAO, will be evaluated for the polymerization of ethylene and propylene. Similar complexes have shown exceptional activity and also extremely high stereoregularity with propylene, if the rac-isomer of the metallocenes is used. 1 The polymers will be characterized by GPC and the stereoregularity will be analyzed by NMR. 5 The rate of the polymerization will also be measured by analysis of amount of polymer formed in a given time period.
B. Other cyclopentadienes
We also intend to utilize reactions similar to those outlined above using the 1,3-substituted cyclopentadienes we have synthesized over the past few years. 6-8 Similar cyclopentadienes have been converted with dimethylsilyl bridges into ligands that yield very active catalysts for the polymerization of alkenes when combined with zirconium fragments. We intend to utilize our ligands such as 1,3-bis(1-methylcyclohexyl)cyclopentadiene or 1,3-biscyclohexylcyclopentadiene in synthetic routes similar to those outlined above for tetrahydroindenes. Again, the catalytic activity will be evaluated.
C. Other spacer groups
The chemistry proposed in the previous two parts of this proposal both utilize the dimethylsilyl fragment as a spacer group between the two tetrahydroindene or substituted cyclopentadiene moieties. It would be interesting to investigate the effect of using other spacer groups. Complexes involving an ethylene bridge or two carbon atom bridge (-CR 2CR 2-; R = H or other group) have been made and many of these exhibit good activity as catalysts. One major problem involved in extending these methods to substituted cyclopentadienes is that several different products are possible. This limits the utility for catalyst synthesis as several different molecules could be produced. We intend to make use of the dimerization of fulvenes. Several metals are known to facilitate this transformation to yield molecules having a two carbon bridge between the two cyclopentadiene rings, but in this case only a single molecule results. 9 This method represents a great improvement over the methods used with unsubstituted cyclopentadienes. We already have developed routes to a wide variety of fulvenes with different substituents which when dimerized will lead to several novel ligands.
References
- H.H.Brintzinger in W. Kaminsky and H. Sinn (eds.) Transition Metals and Organometallic as Catalysts for Olefin Polymerization, Springer-Verlag, Berlin, 1988, 249-256.
- S.Collins, B.A.Kurtz, N.J.Taylor, and D.G.Ward, J. Organometal.. Chem.1988, 342, 21.
- R.N. Austin, T.J. Clark, T.E. Dickson, C.M. Killian, T. A. Nile and D.J. Schabacker, J. Organometal. Chem., 1995, 491, 11.
- S.Dev, J. Ind. Chem. Soc., 1957, 34, 169.
- J. A.Ewen, J. Am. Chem. Soc.1984,106, 6355.
- T.J. Clark and T.A. Nile, Polyhedron, 1989, 8, 1804.
- T.J. Clark and T.A. Nile, Synlett, 1990, 589.
- T. J. Clark, C. M. Killian, S. Luthra and T. A. Nile, J. Organometal. Chem., 1993, 462, 247.
- M. Riekehoff, U. Pieper, D.Stalke and F. T. Edelmann, Angewandte Chemie, Int. Ed., 1993, 32, 1079.





